

| Samyang Holdings Biopharmaceuticals Division Acquires Sales Approval in Japan for Its Anti-Cancer Drug Azacitidine Injection 페이스북 트위터 Print |
|---|
| Affiliates Samyang Holdings Writer administrator Hits 1122 Date 2022.03.17 |
|
- Azacitidine Inj., a treatment for MDS, exported to Japan - First generic drug released in two volumes, 100 mg and 150 mg, with exceptional technological development and production competence - Plans to aggressively target Japanese market with advantages of first generics and diversified volumes
▲ 100 mg (left) and 150 mg vials of Azalid Inj. made with Azacitidine for MDS treatment, released in Korea in 2018 by Samyang Holdings Biopharmaceutical Division
Samyang Holdings Biopharmaceuticals Division (Head Lee Young-Joon) is to enter the Japanese blood cancer drug market.
On the 18th, Samyang Holdings announced that it acquired sales approval for 100 mg and 150 mg vials of Azacitidine Injection, treatment for myelodysplastic syndromes (MDS), from Japan’s Ministry of Health, Labor and Welfare. MDS are a group of rare blood cancers that are rare and intractable in which anomalies occur when the bone marrow makes blood and the numbers and functionality of red and white blood cells and platelets drop below normal levels.
Following this approval, Samyang Holdings Biopharmaceuticals Division will be exporting its products manufactured in Dajeon Plant and Nippon Kayaku, Japan’s no. 1 pharmaceutical company in generic anti-cancer drugs market, will undertake the distribution including marketing and sales as its local partner.
Having secured stable production as well as development capabilities including clinical tests and regulatory affairs, Samyang Holdings Biopharmaceuticals Division successfully released its first generic drug through contract development and manufacturing organization (CDMO) method. This is the second time Samyang Holdings Biopharmaceuticals Division released its products in Japan employing CDMO, after Oxaliplatin Injection, a treatment for solid cancers.
Due to its complicated manufacturing process, there are only a few companies that can stably manufacture Azacitidine Injection. In Korea, Samyang Holdings Biopharmaceuticals Division was the first to successfully localize it in 2018 and began exportation to the EU nations since last year.
According to IQVIA, a global pharmaceutical market research company, the market size of Azacitidine Injection in Japan was KRW 140 billion last year. Samyang Holdings Biopharmaceuticals Division plans to expand its supplies to reach 35% market share in Japan.
Samyang Holdings plans to aggressively target Japanese market with advantages of first generics and diversified volumes of 100 mg and 150 mg vials. Until recently, there was only the 100 mg vial which led to most of drugs being discarded after a single use, depending on the patient size. Anti-cancer drug dosage is determined by the body surface area of the patient. Diversified volumes means less financial burden on the patients and ease of prescription for the medical staff.
An official from Samyang Holdings Biopharmaceutical Division said, “Following the EU approval in 2020, we got approval by Japan, a country from which getting an approval is complicated, which boosted our international credibility. With our market entry into pharmaceutically advanced countries, we will continuously expand our exports to Southeast Asia, Middle East and Central and South Americas.”
Samyang Holdings Biopharmaceutical Division is the only company in Korea to retain GMP certificate from both the EU and Japan for cytotoxic anticancer injection drug. It is currently exporting APIs and drug products to pharmaceutically advanced countries such as Germany and Japan as well as around 30 countries. In order to promote global CDMO projects, Samyang is expanding its cytotoxic anticancer injection drug plant. Currently, Samyang is expanding its Daejeon plant to be able to manage a total of 5 million vials — 4 million injection vials and 1 million lyophilized injection vials. |
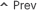 |
Samyang Group’s Yang Young and Sudang Foundations Deliver Scholarship Funds 2022.03.17 |
|---|---|
 |
Samyang Packaging Prioritizes ESG by Expanding Its Plastic Recycling Business 2022.01.25 |